Air & Gas volume conversions
Description
Air and gas volume conversions are often needed when working with different units or conditions. Here are some common conversions between different units of volume and how to convert gas volumes from one set of conditions to another:
- Volume unit conversions:
1 cubic meter (m³) = 1,000 liters (L) 1 liter (L) = 1,000 milliliters (mL) 1 cubic foot (ft³) = 7.4805 gallons (gal) 1 gallon (gal) = 3.7854 liters (L)
To convert between different units of volume, you can use simple multiplication or division. For example, to convert 100 cubic feet to liters:
100 ft³ * (7.4805 gal/ft³) * (3.7854 L/gal) ≈ 2,831.7 L
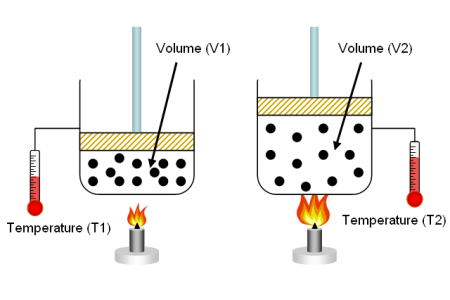
- Converting gas volumes between different conditions:
When working with gases, it's often necessary to convert volumes between different temperature and pressure conditions. To do this, you can use the Ideal Gas Law or the general gas law, which states:
P1 * V1 / T1 = P2 * V2 / T2
where:
- P1 = initial pressure
- V1 = initial volume
- T1 = initial temperature (in Kelvin)
- P2 = final pressure
- V2 = final volume
- T2 = final temperature (in Kelvin)
Keep in mind that temperatures must be in Kelvin (K) for this equation. To convert Celsius to Kelvin, add 273.15 to the Celsius temperature.
Example: Convert the volume of a gas from 50 L at 20°C and 1 atm to the volume at 10°C and 1.5 atm.
First, convert the temperatures to Kelvin: T1 = 20°C + 273.15 = 293.15 K T2 = 10°C + 273.15 = 283.15 K
Next, use the general gas law to find the final volume (V2): (1 atm * 50 L) / 293.15 K = (1.5 atm * V2) / 283.15 K
Solve for V2: V2 ≈ 32.1 L
In this example, the gas volume would be 32.1 L at 10°C and 1.5 atm. Remember that the Ideal Gas Law is an approximation, and the accuracy of volume conversions will depend on the specific gas and the range of conditions. For high pressures or low temperatures, more accurate models like the van der Waals equation or the Peng-Robinson equation may be required.
Calculation Preview
Full download access to any calculation is available to users with a paid or awarded subscription (XLC Pro).
Subscriptions are free to contributors to the site, alternatively they can be purchased.
Click here for information on subscriptions.